The Industry Challenge
Increasing Compliance is challenging Pharma companies efficiency.
Every GMP-Compliant site spends hundreds of hours reviewing and updating its documentation — across multiple systems and teams.
Manual validation consumes experts time, creates silos, and increases the risk of inconsistencies before audits or inspections.
As the industry scales, so does the burden of documentation. Pharma AI was designed to remove that bottleneck — turning manual compliance into intelligent automation.
In just minutes, Pharma AI detects gaps, inconsistencies, and outdated information, delivering a ready-to-review analysis and validation for the audit report.
How It Works
Fast, compliant and precise in only two steps.
Compliance, Security & Trust
Designed for the world's most regulated environments.
Secure & Validated by Design
Built with OWASP-aligned secure coding and verified through independent penetration testing, ensuring system robustness and data protection.
Audit-Ready at
Every Step
Maintains complete audit trails for every analysis, document version, and system interaction — guaranteeing traceability from input to output.

Controlled Access & Accountability
Compliance isn't a feature. It's the foundation, deeply embedded into every action, decision, measurable output, and secured end-to-end with mandatory MFA.
Enterprise-Grade & Compliant
Operates under privacy-by-design principles, ensuring GDPR compliance, and a validated AI infrastructure built specifically for regulated industries.
Use cases
One platform. Three essential GMP applications.
Supplier Qualification
Analyze supplier SMFs to verify GMP alignment and assess compliance before entering the qualification process. Conduct a risk-based review of procedures, controls, and certifications to ensure supplier reliability and regulatory conformity. Establish clear approval criteria to determine supplier eligibility with full transparency.
Internal Audit Preparation
Detect outdated data and ensure full GMP alignment before internal audits. Review procedures, records, and operational controls to confirm they are current, approved, and implemented as described in the Site Master File. Identify gaps or deviations early and apply corrective actions with full traceability to strengthen internal audit readiness.
Regulatory Inspection Readiness
Validate your Site Master File to ensure it accurately represents current operations and is fully aligned with GMP requirements. Confirm that procedures, records, and practices described in the SMF are implemented consistently across the site. Identify discrepancies early and update documentation proactively to ensure full readiness for Health Authority inspections.
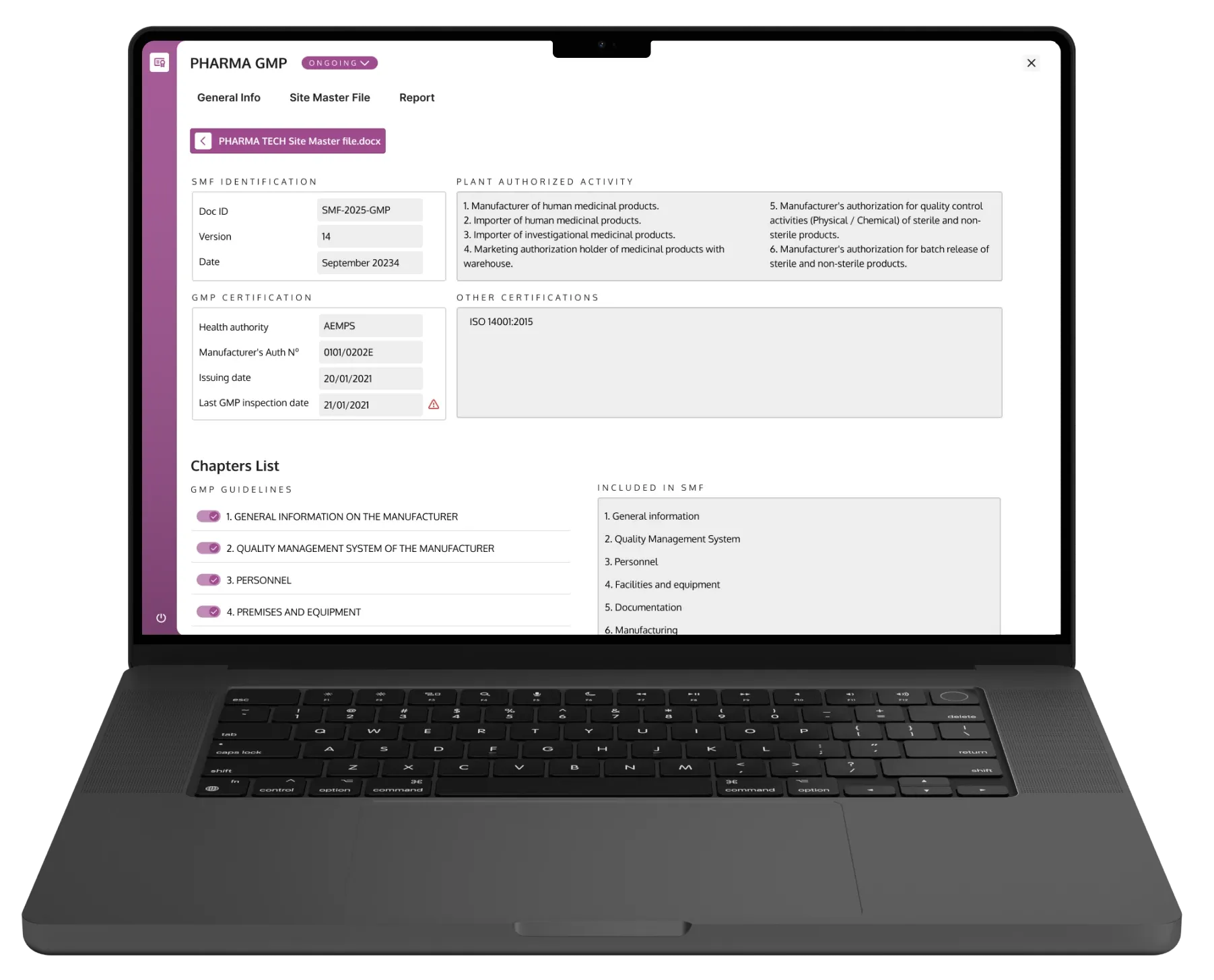
Pharma AI helps pharmaceutical manufacturers accelerate GMP documentation verification and ensure continuous compliance.
Upload the existing Site Master File and let AI automatically identify gaps, inconsistencies, and outdated information — reducing audit preparation time, human error, and inspection risk.